PHARMARINE plans field sampling of water and marine biota, and a series of laboratory experiments on the effects of human pharmaceuticals on key Arctic macrobenthic faunal species using laboratory facilities available on Spitsbergen. The project employs thus interdisciplinary approach combining expertise in different scientific fields including oceanography, marine chemistry and ecology, toxicology, physiology and genetics.
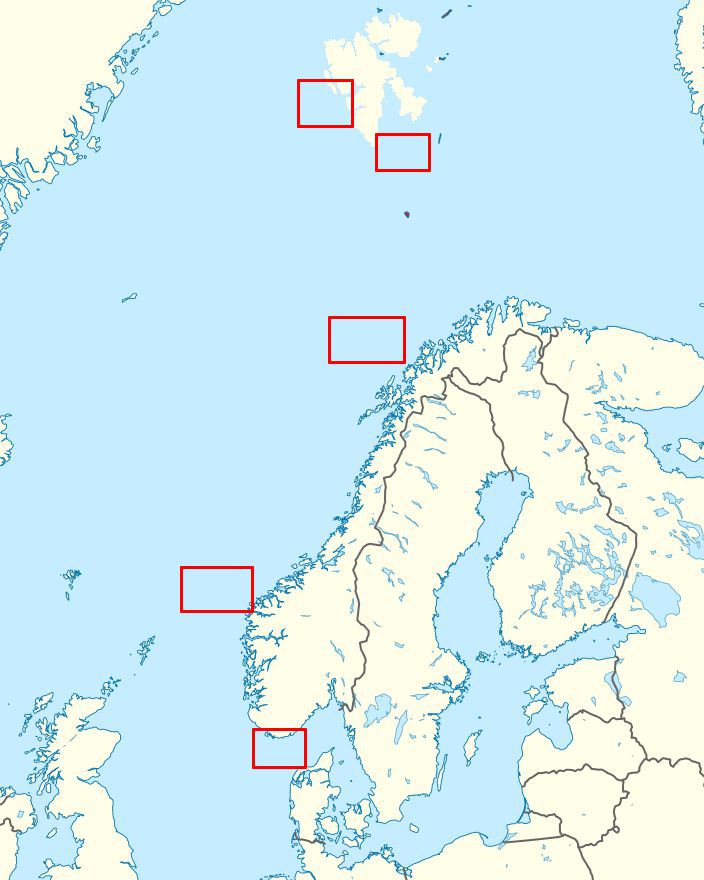
Fig. 1 Sampling locations (two sites are planned in each location)
Within realization of research tasks in WP1, potential carriers of drugs (water and dominant zooplankton species) will be collected in two water layers (surface and subsurface) at five locations (two sites each) (Fig. 1) representing coastal and offshore waters in the outlet of the Danish Straits to the North Sea, coastal and offshore waters along the Scandinavian Peninsula, and coastal and offshore waters in the European Arctic of the Svalbard Archipelago.
Water mass transport over this area is governed by the Norwegian Coastal Current and the North Atlantic Current and its derivatives (Norwegian Atlantic Current, West Spitsbergen Current), as well as the Sørkapp Current in the Svalbard region (Fig. 2). Since concentration of pharmaceuticals in seawater and zooplankton is likely to be very low, a large body of water will be pumped through the LV SPE system. Concentration of pharmaceuticals in the field samples will be analysed using LC-MS/MS (Liquid Chromatography coupled with tandem Mass Spectrometry). Pseudo-discovery, in which a large list of pharmaceuticals is analysed semi-quantitatively, will be applied to identify the most relevant/highly abundant pharmaceuticals in the matrices investigated.
The initially chosen model pharmaceuticals (Table 1) include four compounds of different biological effects and activity (Table 1).
Compound Class Acronym Structure pKa¹ Log Kow² Diclofenac nonsteroidal anti-inflammatory (NSAID) DCF 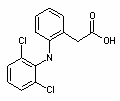
4.00 4.26 Fluoxetine antidepressant FLX 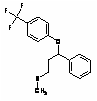
9.80 4.17 Simvastatin lipid lowering medication SMV 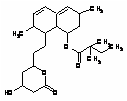
14.91 6.03 Tetracycline antibiotic TCN 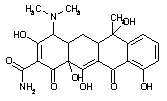
3.30 -1.33
1 acid-base dissociation constant, 2 octanol-water partition coefficient
Laboratory studies: The partitioning of pharmaceuticals among seawater and zooplankton will be investigated through laboratory studies using four model pharmaceuticals. The experimental setup for partitioning experiments will involve different combinations of seawater, inorganic and organic particles and phytoplankton spiked and equilibrated with different pharmaceuticals. The different matrices will be sampled, extracted and analysed as described above. The cold-water copepod Calanus finmarchicus of developmental stage V from the laboratory culture of SINTEF Ocean SA will be used to determine the uptake and depuration kinetics of the drugs. The setup will be continuous flow through exposure during the uptake phase and continuous flow of clean seawater in the depuration phase. It is tentatively planned 6 days of uptake and 6 days of depuration with regular sampling of exposure solutions and zooplankton biomass. Water samples and zooplankton biomass will be extracted and analysed for the target compounds.
Within WP2, benthic fauna will be sampled in two Arctic areas differing in hydrological, climatological and ecological characteristics and potentially in pharmaceutical contamination level: (1) Hornsund, a fjord remaining under influence of the cold Sørkapp Current carrying Arctic waters from the eastern part of the Svalbard archipelago and (2) Isfjorden, a fjord under pronounced influence of Atlantic waters from the West Spitsbergen Current, and most likely influenced also by a drug point source from the local community. Due to apparent impact of pharmaceutical sources from a small community and the airport in Longyearbyen, two separate sampling sites are planned: one in Isfjorden and one in Adventfjorden in order to distinguish between the influence of Atlantic water masses and local effluents. Additionally, samples of suspended particulate matter, surface sediments and selected benthic and pelagic taxa representing different ecological formations and trophic levels will be taken at one Arctic location (Isfjorden). All materials collected during the field campaign in the Arctic fjords will be analysed for concentrations of pharmaceuticals using such techniques as ASE (Accelerated Solvent Extraction), SPE (Solid Phase Extraction) and LC–MS/MS. The results obtained will:
(1) provide additional support to assessment of potential role of aqueous horizontal transport and local sources in the presence of pharmaceuticals in the Arctic
(2) allow defining contamination status of the Arctic using the resident benthic fauna.
Furthermore, components of benthic food web (i.e., potential carbon sources and consumers) will be analysed for the nitrogen (15N/14N) isotopic ratios using a continuous-flow isotope-ratio mass spectrometer (Delta V Advantage, Thermo Scientific) coupled to an elemental analyser (Flash EA1112 Thermo Scientific) that provides simultaneous data on carbon (13C/12C) isotopic ratios and carbon and nitrogen contents. By combining data on nitrogen stable isotope ratios (i.e., an indication of relative trophic position in the food web) with pharmaceutical concentrations, trophic transfer of the drugs will be estimated.
Based on the abundance structure of the macrobenthic faunal community in Hornsund and Isfjorden, and the results of pharmaceutical analyses, exposure experiments will be conducted on two dominant benthic taxa using the laboratory infrastructure available in the Arctic. Within WP3, biological impacts and environmental risks associated with exposure of marine fauna to selected pharmaceuticals will be determined using macrofaunal species which play a key role in the functioning of benthic ecosystem in coastal waters of Spitsbergen. The choice of model species will be made based on data on density and biological traits of faunal taxa which predominate in benthic communities in two Arctic fjords (Hornsund and Isfjorden) available in relevant literature and archives of the PHARMARINE partners. Cause-and-effect relationships of pharmaceuticals and biological responses (biomarkers) will be delineated in field animals which will be collected in two Arctic fjords and those used in laboratory experiments. Short-term laboratory experiments are planned in replicates under temperature-controlled conditions in research town Ny Ålesund located in Oscar II Land on Spitsbergen, Norway. After 3-day acclimatization, the animals will be exposed to different concentrations of two pharmaceuticals using flow-through aquarium system over 10 days. In addition, a long experiment (four-six weeks) on uptake, depuration and kinetics of the same pharmaceuticals by a model zooplanktonic species Calanus finmarchicus, will be run under temperature-controlled-conditions in stationary laboratory in Trondheim to assess biological impacts on longer timescale. The experiment will be divided into two phases, exposure and depuration. For both field and experimental animals, a multistage process will be employed. This approach starts with exposure and progresses through deposited body dose, biologically effective dose, early biological effect, altered structure or function to end with severe health effects (Links et al., 1995). Any stage of this process may be modified by host-susceptibility factors and all of the stages except initial exposure represent processes occurring within the organism. To this end, biologically effective dose markers such as micronuclei, DNA and protein adducts will be used to assess the biologically active fraction of pharmaceuticals which is capable of interacting with cellular macromolecules at the target site (Miraglia et al., 2004). In addition, transcriptomic analysis will be used to study changes in gene activities under the influence of drugs in both field and experimental studies. Transcript levels of stress genes will be quantified using crystal digital PCR (cdPCR) technology which allows absolute quantification. To investigate pharmaceutical toxicity (from biologically effective dose to the disease), biomarkers will be chosen at an advanced stage of usage and development reflecting different steps on the dose-effect curve. After exposure to pharmaceuticals, different levels of changes will be measured using specific methods: the biologically effected dose will be reflected by molecular biomarkers and transcriptomics while general health and physiological conditions will be described by nucleic acid derived index RNA/DNA ratio and enzymatic and stress assays. Markers of oxidative stress will include activity of antioxidative enzymes such as catalase, superoxide dismutase and glutathione peroxidase, measurement of the amount of low-molecular weight antioxidants (uric acid, ubichinones, glutathione GSH) and lipid and protein peroxidation products (MDA and CBO). Assessment of changes in the main detoxification pathways will be performed via analyses of phase I detoxification enzymes (cytochrome P450 monooxygenase, monoamine oxidase), phase II detoxification enzymes (UDP-glucuronosyltransferase, glutathione S-transferase, methyltransferase) and phase III detoxification proteins (P-glycoprotein). What is more, histological analyses will be performed to assess health and physiological condition of animals. Changes in physiology will indicate the intensity of metabolic processes and provide physiological responses of organisms to drugs present in the ambient environment. Physiological stress will be measured via assessment of physiological and metabolic patterns (sex ratio, gonadal development, body mass). In addition, genetic polymorphism (e.g. phase I enzymes CYP450) in different species will be analysed.
The PHARMARINE project will be managed by University of Gdańsk (UG), Institute of Oceanography, Poland (WP4). Clear management of the science will be fundamental to the success of the project. The Principal Investigator (PI) will be supported by a Steering Committee (SC) which will consists of the PI, work package leaders and a representative from the participating institutions, and will be chaired by the Principal Investigator. The SC meetings will be held at least once a year in consort with the annual scientific meeting which will gather representatives of all partner institutions. It will report to the Program Operator.